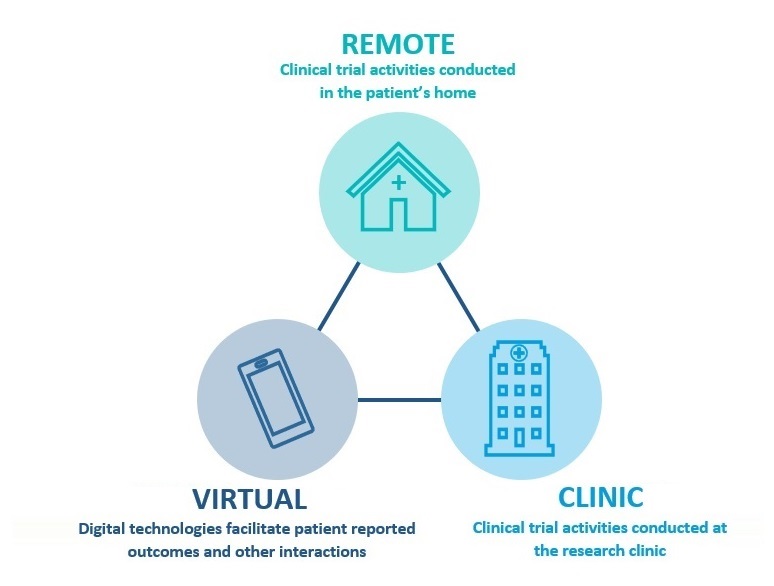
Remote Clinical Trial Support
MAC Remote Trial
MAC’s remote clinical trial model enables many clinical trial activities to be conducted in the patient’s home. MAC Remote Trial reduces the number of visits to the Investigator Site and can significantly lessen the burden on patients and their families. Ease of participation makes a significant difference to clinical trial recruitment (particularly during challenging times) and strongly supports participant retention. MAC has deployed a range of advanced solutions within our digital clinical trial suite to mitigate study challenges.
Pharmacy
MAC can support complex IMP administration, collaborating with Investigator Site pharmacy. IMP can be prepared by MAC’s accredited GMP manufacturing facilities and shipped to the patient, precisely matching the timeframe of the remote study visit (shelf life and logistics permitting. Where appropriate IMP can be shipped and reconstituted in the patients home by the clinical trial team.)
Logistics
MACs logistics supply chain maintains quality and is fully accountable, for the transportation, dispensing and storage of clinical trial products. MAC’s broad CRO offering facilitates a seamless integrated approach to full service clinical trial delivery.